What are the IVC Filter Lawsuits All About?
When they first came to light, the Inferior Vena Cava Filters (IVC) were among the smartest inventions of all time. Across the world, people suffer from all kinds of health complications. When there is finally a solution to all their problems, it's more like a gift from heaven, or is it? Blood clots are a medical condition that affects millions of people around the globe.
The IVC devices were supposed to be a way of preventing the occurrence of the blood clots in the body, which would otherwise result in disastrous complications or even death.
With that said, it's up to the manufacturers to ensure the medical devices they put in the market are safe for use. Sad to say, things did not go as planned with the IVC filter devices manufactured by CR Bard and Cook Medical. There have been claims that the IVC filter devices are defective, with some breaking apart or moving within the body.
Thousands of affected patients have already filed lawsuits seeking compensation for the complications they suffered. According to the IVC filter lawsuits, the manufacturers put a defective medical product into the market and failed to warn the patients about the fatal risks and complications. Without saying too much, let's get into the details;
What we know about the IVC filters
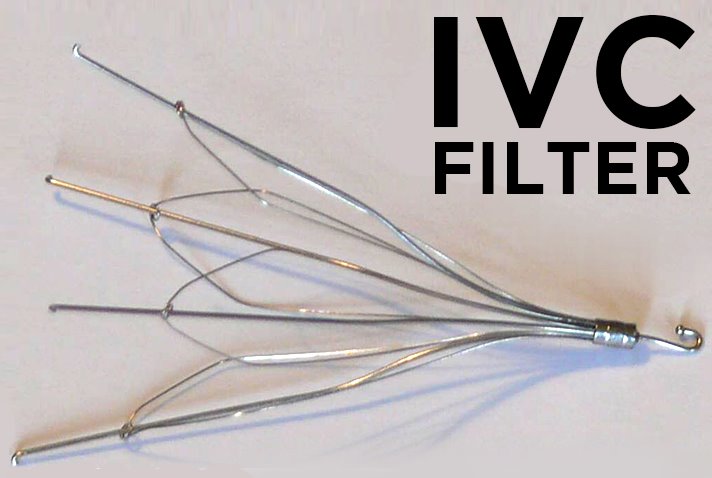
The IVC filters are typically medical devices that work to prevent the formation of life-threatening blood clots in patients. These are mostly the trauma patients who are at high risk of developing blood clots as well as the obese patients. Some of these patients cannot take anticoagulants because they have proven to be ineffective. For this reason, the IVC filter devices are used instead.
A medical professional inserts the device in the neck or groin area and threads it into the inferior vena cava. Well, as you might know, the inferior vena cave is responsible for carrying deoxygenated blood from other parts of the body to the heart. The implanted IVC filter device captures any blood clots moving within the artery before they can get to the lungs and cause problems.
However, the very same devices that were supposed to be preventing disaster were the ones reportedly causing serious complications in patients who used them. As early as 2010, there were patients from different parts of the country who were reporting horrific complications such as the device migrating to other parts, the filter breaking into pieces, punctured blood vessels, and body organs. The FDA took notice of that and issued its first warning the very same year. At the time, the patients were advised to have the implants removed as soon as the risk of blood clots decreased.
IVC Filters issues, removal and complications
IVC filters are not medical devices that are new to the world. They have been here with us since the 1970s, and with time different types of both permanent and temporary filters have been developed. With that said, IVC filters are usually implanted in trauma patients or those who are not responsive to anticoagulant medications (blood thinners).
What happens typically is an IVC filter device is placed within the inferior vena cava to trap any blood clots that may travel to the heart or lungs. The trapped blood clots will then dissipate naturally over time. In this case, I would be correct to say they work to prevent severe health conditions such as pulmonary embolism.
At a glance, IVC filters are quite useful medical devices that have saved the lives of many patients or simply made their lives much more comfortable. However, they also have potential risks and complications that can turn out fatal. In 2010, the FDA received so many reports from people who claim to have suffered filter-related complications.
This prompted the FDA to issue a safety alert on all the filter devices. The patients were advised to have their filters removed between 29 and 54 days after implantation once the risk of developing blood clots had passed.
IVC filter issues
Initially, many of the IVC filter models were intended for permanent use. This basically means that the IVC filter devices were to be left in place permanently; there was no such thing as removing them. Unfortunately, the FDA received numerous reports of adverse events and problems associated with the devices.
Some of the adverse events reports include filter fracture, device migration, the IVC device perforating the vein wall or vital organs, and difficulty removing the devices.
Studies conducted showed the IVC filter complications were likely to arise from the device being left in place for too long. For this reason, manufacturers came up with the newer retrievable types of IVC filters as a means of addressing all these issues. According to the FDA, the retrievable IVC filters ought to be removed as soon as the risk of developing pulmonary embolism subsides.
This will prevent some of the complications from occurring while the newer filters are way more advanced, the risk of complications has not gone down. The problem usually comes with removing the filter, especially if it has remained in place for a prolonged period.
For instance, excessive scarring around the indwelling filter may mean higher risks of procedural complications like bleeding, blood clotting, and vessel occlusion. If there are any existing fractured filter fragments, there is a risk of them migrating during the procedure. Thankfully, plenty of research has been done in this field, and there are now lots of minimally-invasive retrieval options for patients with filters.
Have you or a family member suffered complications or serious side effects or death?
If you or a family member has been using an IVC Filter and have any legal questions related to illness or death, contact an attorney right away to discuss your case.